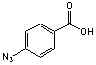
p-Azidobenzoic
acid (99+%)
C7H5N3O2;
Mr=163.14
●
Useful for generating photoaffinity probes for mapping
receptor-ligand complexes.1
●
Introduces carboxylate functionality onto non-derivatizable surfaces,
ie. polystyrene, polypropylene; microtiter plates, beads, etc.
1. Chatreneet, B., et.al. (1990) Proc. Natl. Acad. Sci. USA
87, 3378.
Storage 25 °C.
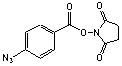
N-Hydroxysuccinimidyl 4-azidobenzoate
[53053-08-0]; C11H8N4O4; Mr=260.21; mp 171-172 °C
●
Amino and photoreactive protein crosslinking reagent.1
● Photoaffinity crosslinking of calmodulin to binding proteins.2,3
1. Vandlan, R.L., et.al. (1985) J. Biol. Chem. 260,
10889-10892.
2. Wood, C.L., et.al. (1985) J. Biol. Chem. 260, 1243-1247.
3. Goewert, R.R., et.al. (1982) Biochemistry 21, 5310.
Storage 0-4 °C.
p-Azidobenzoylhydrazide; 99%
C7H7N5O; Mr=177.17; mp 169-170 °C
Spacer Arm Length: 11.9 Å.
●
Photoreactive heterobifunctional crosslinking reagent.1
● Useful for crosslinking glycoproteins
to antibodies.2
1. O'Shannessy, D.J., et.al. (1985) J. Applied Biochem. 7,
347-355.
2. O'Shannessy, D.J., et.al. (1984) Immunol. Lett. 8, 273-277.
Storage 0-4 °C.
250mg
$200.00
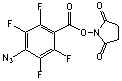
C11H4N4O4F4; Mr=275.17
●
Fluorine groups stabilize intermediate nitrene,
leading to higher yields of intermolecular insertion products.1
● Surface modification reagent for derivatizing unactivated surfaces; i.e.
polystyrene, polypropylene, metal films, glass, DNA, RNA, etc.
1. Keana J. F. W., Cai, S. X. (1990) J. Org. Chem. 55, 3640-3647.
Storage 0-4 °C.
●
Water soluble!
●
Fluorine groups stabilize intermediate nitrene,
leading to higher yields of intermolecular insertion products.1
1. Keana J. F. W., Cai, S. X. (1990) J. Org. Chem. 55, 3640-3647.
Storage 0-4 °C.
p-Azidophenylglyoxal monohydrate; 99 %
C8H7N3O3; Mr=193.16; mp 44-46 °C
Spacer Arm Length: 9.3 Å.
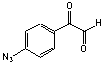
● Arginine and photoreactive heterobifunctional crosslinking
reagent.1
●
Irreversibly modifies arginine side chain residues at pH 7.0-8.0.
Used to crosslink RNA-protein interactions.2
1. Sgro, J., et.al. (1984) Eur. J. Biochem. 154, 69-76.
2. Poltz, S.M., Noller, H. F., McWhirter, P. D. (1981) Biochemistry 20, 372-378.
Storage 0-4 °C.
4-(4-Azidophenyl)butyric acid hydrazide
C10H13N5O; Mr=219.25; mp 86-87 °C
![]()
●
Carbonyl and photoreactive crosslinking reagent.
● Immobilization of glycoproteins through their carbohydrate residues.
Storage 0-4 °C.
250mg
$250.00
[28166-06-5]; C6H3N4FO2; Mr=182.12; mp 53-55 °C
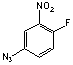
●
Heterobifunctional crosslinking reagent for photoaffinity
labeling.1,2
1. Hagedorn, M., et.al. (1978) J. Org. Chem. 43, 2070-2072.
2. Tometsko, A.M., et.al. (1976) Int. J. Peptide Protein Res. 8, 331.
Storage 0-4 °C.
[57018-46-9]; C8H6N3OBr; Mr=240.05; mp 68-70 °C
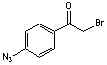
●
Sulfhydryl and photoreactive crosslinking reagent.1
1. Schwartz, I., Ofengand, J. (1974) Proc. Natl. Acad. Sci.
71, 230.
Storage 0-4 °C.
5.0g
$275.00
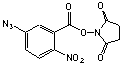
N-(5-Azido-2-nitrobenzoyloxy)succinimide
[60117-35-3]; C11H7N5O6; Mr=305.21; mp 131-133 °C
Spacer Arm Length: 7.7 Å.
●
Amino and photoreactive heterobifunctional crosslinker.1,2
● Photolysis occurs at 320-350 l.
Labeling is usually complete within 15-20 min.
1. Schafer, H. (1987) Chemical Modification of enzymes, ED.
Eyzaguirre, J. 45-62.
2. Krieg, U.C., Walter, P., Johnson, A.E. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 8604-8608.
Storage 0-4 °C.
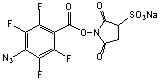
N-(5-Azido-2-nitrobenzamidocaproyl) sulfo-succinimide
C17H17N6O10SNa; Mr=520.41
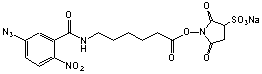
●
Water soluble and extended
analog of ANB-NOS!
● Photolysis occurs at 320-350 l.
Labeling is usually complete within 15-20 min.
Storage 0-4 °C.
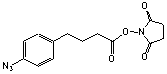
Succinimidyl 4-(4-azidophenyl)butyrate
C14H14N4O4; Mr=302.29
● Converts amines into photoaffinity probes, or surface modification reagents.
Storage 0-4 °C.
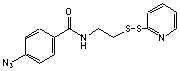
C14H13N5S2O; Mr=331.42
●
Converts sulfhydryls into cleavable photoaffinity probes.
● Used to label granulocyte C5a receptor.1
1. Johnson, R.J., Chenoweth, D.E. (1985) J. Biol. Chem. 7161-7164.
Storage 0-4 °C.
N-Succinimidyl-6-(4´-azido2´-nitrophenyl-amino)hexanoate
[64309-05-3]; C16H18N6O6; Mr=390.95
sulfo-Succinimidyl-6-[(4´-azido2´-nitrophenyl)amino]hexanoate
C16H17N6O9SNa; Mr=492.39
Spacer Arm Length: 18.2 Å.
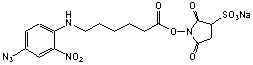
●
Water soluble!
●
Heterobifunctional
crosslinking reagent, Photolysis
occurs at 320-350 nm.1
1. Schmitt, M., et.al. (1983) J. Biol.
Chem. 258, 649-654.
Storage 0-4 °C.
(SAND) 99 %
sulfo-Succinimidyl 2-[m-azido-o-nitrobenzamido]ethyl-1,3´-dithiopropionate
C16H15N6O10S3Na;
Mr=570.51
Spacer Arm Length: 18.5 Å.
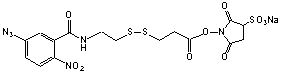
●
Water soluble!
●
Heterobifunctional crosslinking reagent, Photolysis
occurs at 320-350 nm.1
●
Long chain and
cleavable analog of ANB-NOS. Conjugated products can be cleaved with
mild reducing reagents, i.e. DTT, DTE, TCEP.
Storage
0-4 °C.